Philippine Program for Diagnostic Biomarkers, Disease Modeling and Nutriceutical Product Development (Phil-DIAMOND): Initial Focus on HIV-Related Neurocognitive and Metabolic Complications
Component Projects
Project 1: Biomarkers of Immune Dysfunction in HIV-associated Metabolic and Neurocognitive Complications
Project 2: Development of HIV Disease Models and in vitro Investigation of HIV-related Neurocognitive and Metabolic Complications
Project 3: Development of a Safe Lactic Acid Bacteria (LAB)-based Biofunctional Health-promoting Product as an Adjunct Intervention for the Management of HIV-associated Metabolic Complications
Our Mission
“We aim to establish a centralized facility for infectious disease research with initial focus on HIV-related complications with the general aim to investigate immune biomarkers for diagnosis, construct in vitro disease models, and develop probiotic-based functional food as adjunct therapy for HIV related complications among HIV+ Filipino patients.”
HIV infections in the Philippines has been steadily increasing at an alarming rate for the past decade. Despite the use of potent antiretroviral therapy (ART), people with HIV are still vulnerable to the non-infectious sequela of HIV due to persistent viral immune stimulation throughout the body. Viral persistence in the gut also causes an increase in microbial translocation worsening systemic inflammation. This affects different organs resulting in serious non-AIDS-defining events (SNAEs) such as metabolic and neurocognitive disorders. To address this multifactorial problem, this program will establish collaborative, multi-disciplinary research involving characterization of immune cells of HIV+ Filipino patients, disease modeling of SNAEs at a molecular level, and development of probiotic-based functional food to improve metabolic health among HIV+ Filipino patients with SNAEs. For this phase we aim to:
- Enhance infectious disease and HIV research capacity in the Philippines through the establishment of joint facilities and training of Filipino researchers in preclinical and clinical research techniques.
- Profile and correlate biomarkers in Filipino HIV+ patients to HIV-associated complications and investigate causation at molecular level in order to expose novel therapeutic targets and provide preclinical evidence.
- Formulate and develop lactic acid bacteria-based biofunctional food as an adjunct therapy for HIV-related infectious and metabolic complications
Program Framework

To address the need for high-impact and relevant research on pathogenic viruses such as HIV, this program proposes to establish three facilities. Each facility will fulfill the following tasks: (1) to diagnose biomarkers in Filipino HIV+ patients and produce patient-based data in the form of immunophenotype profiles, (2) to model and investigate the molecular mechanisms occurring during progression of HIV-related complications and produce in vitro data for efficient therapeutic targets, and (3) to develop probiotic-based functional food products for potential adjunct therapy in patients suffering from HIV-related complications.
Program in Action
August 2021 Monthly Monitoring Meeting
July 2021 Monthly Monitoring Meeting
Contact Us
Dr. Ana Joy Padua
Phil-DIAMOND Project 1
phildiamond.upm@up.edu.ph
Our Mission
"We aim to investigate how the host’s immune and genetic factors influence neurocognitive and metabolic complications among Filipino HIV+ patients"
The country has been experiencing an alarming increase of HIV cases. And while the response of making ART (antiretroviral therapy) more accessible to patients has restored lifespan and made HIV a chronic stable disease, multi-morbidity associated with HIV has become a pressing concern. Therefore the study aims to characterize neurocognitive and metabolic complications among Filipino HIV+ patients in the treatment hub of the UP-Philippine General Hospital and to investigate the relationship of these complications with CSF and peripheral blood immune dysfunction markers, genetic mutation and detection of viral persistence
More specifically,
- To derive the normative neuropsychological (NP) performance among healthy HIV-negative control Filipino using the battery of tests designed for HAND
- To compare the expression level of 1) NCRs and their cognate ligands, 2) GLUT1 and 3) HIV DNA in PBMCs and CSF from pre-ART (treatment naive) group, 6 months post-ART (virally suppressed) group, with HIV negative healthy control, and examine its relationship with NP performance and metabolic complications among HIV+ Filipino patients in the HIV Clinic of UP-PGH
- To identify potential exonic genetic variations of immune cells related to HIV infection among Filipinos
- Enhance immunogenetics HIV research capacity at the University of the Philippines
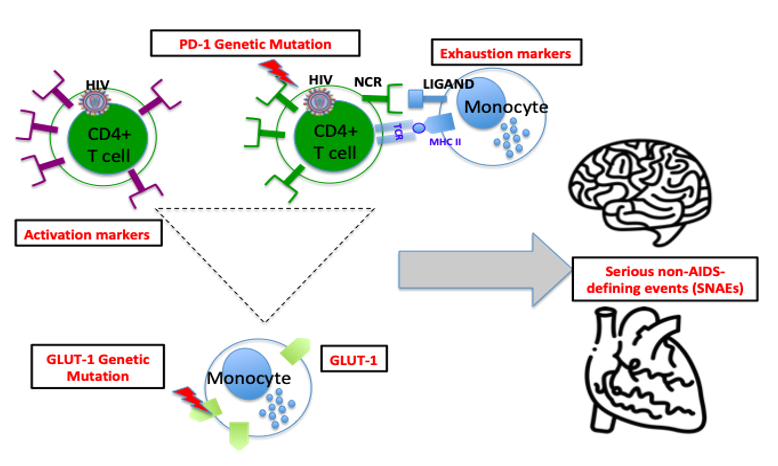
Role of Peripheral and Central Immune Cells in SNAEs: Immune dysfunction, characterized by overexpression and genetic variations of the negative checkpoint Receptors (NCRs) and their cognate ligand, could contribute to the development of serious non-AIDS defining events such as HIV-associated neurocognitive disorder or metabolic disorder.
Meet our Team
 |
Marissa M. Alejandria, MD, MSc, FPCP, FPSMID Principal Investigator |
 |
Ana Joy Padua, MD, PhD Co-Investigator |
 |
Christian Francisco, MD, FPCP, FPSMID Co-Investigator |
 |
Sheriah Laine M. de Paz-Silava, MD, PhD Co-Investigator |
 |
Paul Pasco, MD, MSc Co-Investigator |
 |
Krizelle Julie Ann P. Berago, RMT Science Research Specialist I |
 |
Sharrah Kate Peñaflorida, RPm Science Research Assistant |
 |
Armand Christopher Caneja Project Development Officer |
Project Instruments

Cytoflex LX
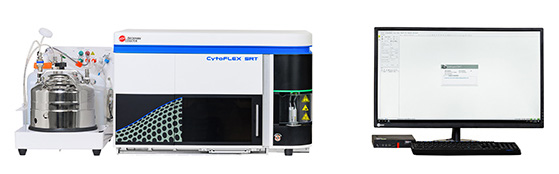
Cytoflex SRT
Flow cytometry is a technique that can be used to measure multiple physical characteristics of a single cell such as granularity and size. Simultaneously, it could measure various cellular events (ex. Cell cycle), intracellular markers (cytokines) and surface markers (receptors and ligands). In this project, the flow cytometry analyzer and sorter are used to analyze immune cells isolated from HIV+ Filipino patients (immunophenotyping). Through this, immune biomarkers related to HIV infection could be developed for future clinical applications.
QuantStudio 3 Real-time PCR system

Quantitative PCR quantification is a highly sensitive technique that can be used to measure specific genetic material in any pathogen. Through this, HIV DNA and RNA will be quantified from isolated immune cells in order to assess the possible relationship of HIV-infected immune cells with HIV-related complications such as neurocognitive and metabolic disorder.
Recent Publications
Chew GM, Chow DC, Padua AJP, et al. Effects of Brief Adjunctive Metformin Therapy in Virologically Suppressed HIV-Infected Adults on Polyfunctional HIV-Specific CD8 T Cell Responses to PD-L1 Blockade. AIDS Res Hum Retroviruses. 2021;37(1):24-33. doi:10.1089/AID.2020.0172
Contact Us
Dr. Ana Joy Padua
Phil-DIAMOND Project 1
phildiamond.upm@up.edu.ph
Our Mission
“We aim to develop biological disease models which recapitulate cellular interactions during HIV-infected complications and use these models to test potential therapeutic drugs/compounds”
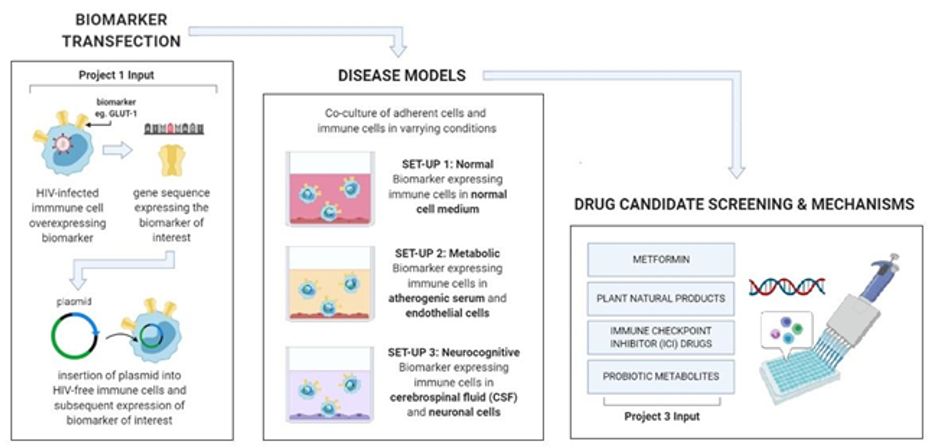
This project aims to analyze the molecular processes involved in HIV-related neurocognitive and metabolic complications using biological models. Due to the limitations of using patients as actual research models, small-scale in vitro models are necessary to understand intimate molecular processes that contribute to the disease itself. Using transgenic technology, it is now possible to transform various human cell lines that mimic the genetic profile and physiological symptoms characterizing the disease as observed in patient cells. Through studying these models in controlled conditions, we can expose critical molecular pathways or checkpoints that can be targeted by synthetic drugs or compounds derived from natural products.
This project will use biomarker data from the diagnostic immunophenotyping of Project 1 as foundation to construct accurate disease models for HIV-related complications. These models will then be applied to study molecular pathways affected by known drugs, chemoregulators and bacterial metabolites from Project 3.
More specifically, the project aims to
- To establish the disease modeling facility at the Institute of Biology, UP Diliman;
- To generate stable immune cells with inducible genes for the candidate biomarkers and establish an in vitro monocyte-endothelium model for atherosclerosis and a lymphocyte-neuronal model for neuroinflammation using commercially available cell lines;
- To generate endothelial and neuronal cells from peripheral blood cells of Filipino patients using induced pluripotent stem cell (iPSC) technology;
- To investigate the role of GLUT-1 and PD-1 overexpression in immune dysfunction leading to atherosclerosis and neuroinflammation;
- To produce pre-clinical data for the use of commercially available drugs metformin and PD-1 inhibitor in circumventing HIV-related metabolic and neurocognitive complications;
- To isolate compounds from plant extracts and probiotic lactic acid bacteria metabolites with therapeutic potential for circumventing immune dysfunction and HIV-related complications;
- To strengthen infectious disease modeling research in the country through facility enhancement and capacity building at University of the Philippines and Mindanao State University;
Project Framework
Meet our Team
 |
Ahmad Reza F. Mazahery, PhD Project Leader Assistant Professor Institute of Biology UP Diliman Principal Investigator Stem Cell In vitro Applications in Research and Modeling (SCIARM) Laboratory |
 |
Mylah V. Tabelin, PhD Project Staff II Professor Iligan Institute of Technology Mindanao State University Principal Investigator Developmental Biology Laboratory |
 |
Sheriah Laine M. de Paz-Silava, MD, PhD Project Staff II Assistant Professor College of Public Health UP Manila |
| Francis James A. Gordovez, MD, PhD Project Staff II Assistant Professor College of Public Health UP Manila |
|
 |
Javier G. Lozano, PhD Project Staff II Visiting Professor Institute of Biology UP Diliman |
 |
Ranelle Janine Asi Senior Science Research Specialist Institute of Biology UP Diliman |
 |
Maevel M. Romero Science Research Specialist II Institute of Biology UP Diliman |
 |
Rod Vincent L. Borromeo, MSc Science Research Specialist II Institute of Biology UP Diliman |
 |
Anna Isabel Navarro Project Development Officer II Institute of Biology UP Diliman |
 |
Katherine D. Walo Laboratory Technician Institute of Biology UP Diliman |
 |
Josephine M. Cano Laboratory Aide II Institute of Biology UP Diliman |
 |
Al James A. Manua Project Development Officer I Iligan Institute of Technology Mindanao State University |
 |
Florifern C. Paglinawan Laboratory Technician Iligan Institute of Technology Mindanao State University |
Project in Action
Bi-weekly journal club. Project personnel and collaborating students present current studies relevant to the project.
Cell culture maintenance. Immortalized cells are kept alive and growing in the laboratory with the help of specialized media and culture manipulation techniques.

Cells for disease models. Immune cells (left and right images) will be transfected with selected genes and will be co-cultured with neuronal cells (middle) to simulate the cellular interactions during neurocognitive diseases.

The search for antiviral compounds from plants with medicinal properties. Crude extracts from plant samples are separated through chemical fractionation methods. Each resulting fraction will be tested using our cellular models to check for antiretroviral properties. This process will be repeated until a pure compound is obtained and identified.
Publications
Almenario FM, Asi RJ, Jacinto SD, Mazahery AR. Reducing mitomycin-C-induced ROS levels in mouse feeder cells improves induced pluripotent stem cell colony growth. Biotechniques. 2020 May;68(5):270-274. doi: 10.2144/btn-2019-0118. Epub 2020 Jan 15. PMID: 31939319.
Asi RJL, Badua CLD, Atun JBD, Lapus JIL, SMO Lopez, Jacinto SD, Mazahery ARF and Balolong MP. An optimized de Man Rogosa Sharpe-McCoy’s 5A medium formulation for the in vitro screening of cytotoxic effects by probiotic candidates against colorectal cancer cells. Philipp Sci Lett 2020; 13(2):139-147.
Project Instruments
Transfection System
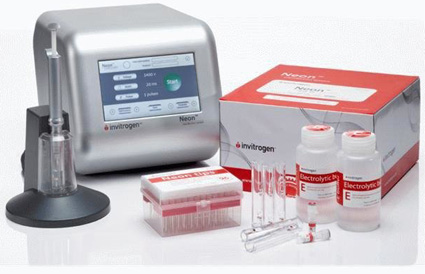
Image source: https://www.thermofisher.com/ph/en/home/life-science/cell-culture/transfection/neon-transfection-system.html
A transfection system is used to insert or transfer genetic material into a cell to study gene function or expression. The Neon Transfection System (Thermo Fisher Scientific) is an electroporation system (physical transfection using electric pulses) employing a conductive pipette tip chamber that is suitable for immune, primary and stem cells. It can transfect as minimal as 2 x 104 cells and uses a single reagent kit for all cell types.
The Project team uses the equipment to transform HIV-free immune cells by insertion of genes overexpressed in HIV-infected cells.

Image source: https://www.benchmarkscientific.com/product/t5000-group/
DNA Analysis (PCR, AGE)
Analysis of DNA of the Project is done with the use of a conventional PCR Machine (Benchmark T5000-96E TC9639) which amplifies or replicates DNA samples and an Agarose Gel Electrophoresis system (Accuris E1201-E) which separates DNA strands.
The Benchmark TC9639 is a thermal cycler with a multi-format block accommodating 0.2mL tubes, 0.2mL strips, a 96 well plate and 0.5mL tubes to analyse samples. The machine has a color touch screen, a Program wizard for setting protocols and a temperature-adjustable lid.
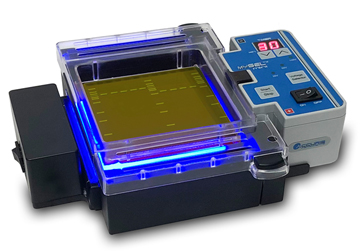

Images source: https://www.accuris-usa.com/Products/mygel-instaview-systems/
The Accuris Instaview accommodates 10.5 x 10 cm or 10.5 x 6 cm, 0.5 cm thick gels. It features a blue LED illuminator for viewing of bands even during the electroporation process and an orange filter lid to allow taking of images without removal of the gel.
Protein Analysis (Westernblot System, PAGE)
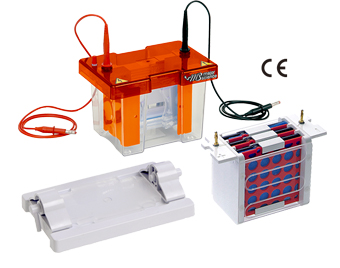
Image source: https://www.majorsci.com/product/6-1-mv-cbs?utm_source=ICM&utm_medium=referral
Analysis of proteins is done with the use of Westernblot Systems. The Major Science Complete Mini Electro Blot System (MV-10CBS) is a complete system for electrophoresis and blotting of proteins using a single buffer tank that allows interchange of the electrophoresis and electroblot modules. The Electrophoresis module is in a vertical format and comes with cooling packs. The Electroblot module can accommodate 4 plates and comes with hinged and lockable cassettes.
Microplate Reader

Image source: https://www.accuris-usa.com/Products/microplate-absorbance-reader/
Microplate readers quantify processes, properties or samples / analytes (viable cells, proteins, nucleic acids etc.) with the use of spectro or optical signals. The Project uses the Accuris SmartReader 96 (MR9600-E) which can accommodate a 96-well plate, operates within 340-750nm wavelength range with the use of filters and allows incubation with temperature control. The machine can be connected to a computer / laptop or it can be a stand-alone unit with a touch screen for programming. Its program allows labelling of wells, curve fitting and kinetic calculations.
Contact Us
PhilDIAMOND Project 2
phil-diamond.ib.updiliman@up.edu.ph
Location: Room 264-268 (MCCL), Institute of Biology, UP Diliman
Hours of operation: 9am-5pm (Mon-Fri)
Our Mission
“We aim to develop a safe biofunctional food supplement as a possible adjunct therapy for the management of metabolic complications in patients with HIV.”
Microbial community dysbiosis has been shown to manifest in HIV+ patients and the use of cART does not alleviate this. Adding probiotic supplements to the diet of HIV+ patients may improve gut health and help manage complications. With the use of two local novel strains of lactic acid bacteria (Lactobacillus plantarum BS25 and Pediococcus acidilactici 3G3), previously isolated from traditional Filipino fermented foods that have been found to lower cholesterol in pre-clinical trials and produce health-promoting compounds such as bacteriocins, Phil-DIAMOND Project 3 aims to develop a safe biofunctional food based on lactic acid bacteria (LAB), and evaluate its safety as a possible adjunct therapy for HIV-infected patients with metabolic complications.
More specifically,
- To screen for biofunctional or health-promoting properties of the two LAB strains addressing HIV-related gut and metabolic complications (diarrhea, dyslipidemia);
- To design the appropriate formulation and preparation of the LAB product with the target biofunctional properties;
- To evaluate the safety, stability and shelf life of the developed product;
- To describe the mechanism behind the biofunctional properties of the developed functional food using a combination of in vitro, in vivo and -omic platforms.
Project Framework
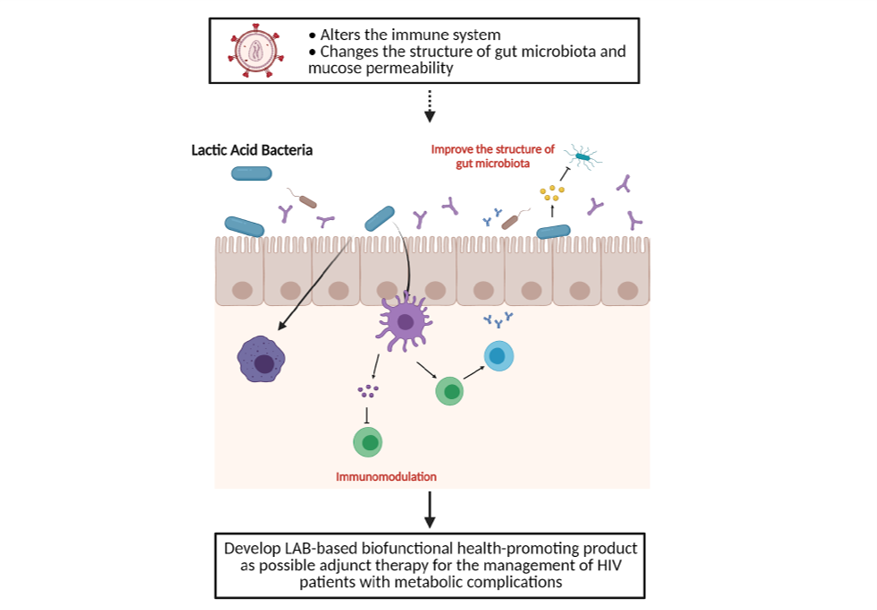
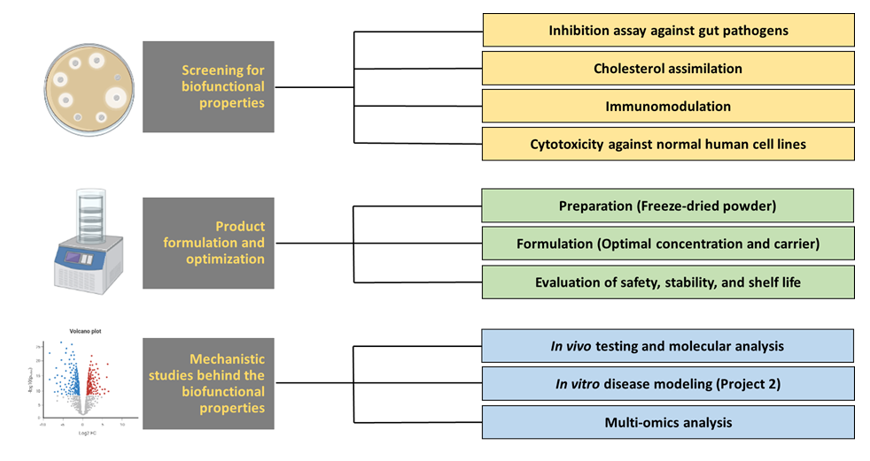
Microbial community dysbiosis particularly in the gut have been suggested to play a potential role in loss of homeostasis leading to the development of systemic infections in patients with HIV. Possible impacts of microbe-based therapies in restoring gut mucosal immunity, as well as their translational potential to supplement current HIV cure efforts, are then necessary to be explored. This project will then harness the potential of LAB strains from Philippine traditional fermented foods and develop a biofunctional product as an adjunct intervention for people living with HIV during the management course of their disease.
Meet our Team
 |
Marilen Parungao Balolong, MSc DrPH DPAM Project 3 Leader Professor of Microbiology, University Scientist College of Arts and Sciences, UP Manila Lead Researcher Applied Microbiology for Health & Environment Research Group (AMHERG) |
 |
Leslie Michelle M. Dalmacio, PhD DPAM Project Staff III Professor of Molecular Biology and Biochemistry College of Medicine, UP Manila |
 |
Francisco B. Elegado, PhD DPAM Project Staff III University Researcher for Agriculture and Biotechnology National Institute of Molecular Biology and Biotechnology (BIOTECH-UPLB) |
 |
Mia Beatriz C. Amoranto, MSc RMicro Science Research Specialist II |
 |
Joseph Q. Bechayda Science Research Specialist II |
 |
Zachary B. Lara Science Research Specialist I |
 |
Princess Alyssa S. Abid University Research Associate II |
 |
Ezekiel H. Florencio Laboratory Aide II |
Project in Action
Bi-weekly journal club. Project personnel and collaborating students present current studies relevant to the project.
Training undergraduate and graduate students. In-house training on primer sequence design to target specific lactic acid bacteria strains using polymerase chain reaction.
Preparation of the two local LAB strains. The project has revived the probiotic strains that will be used for the development of functional food. These two strains will be screened for their inhibitory activities against gut pathogens.
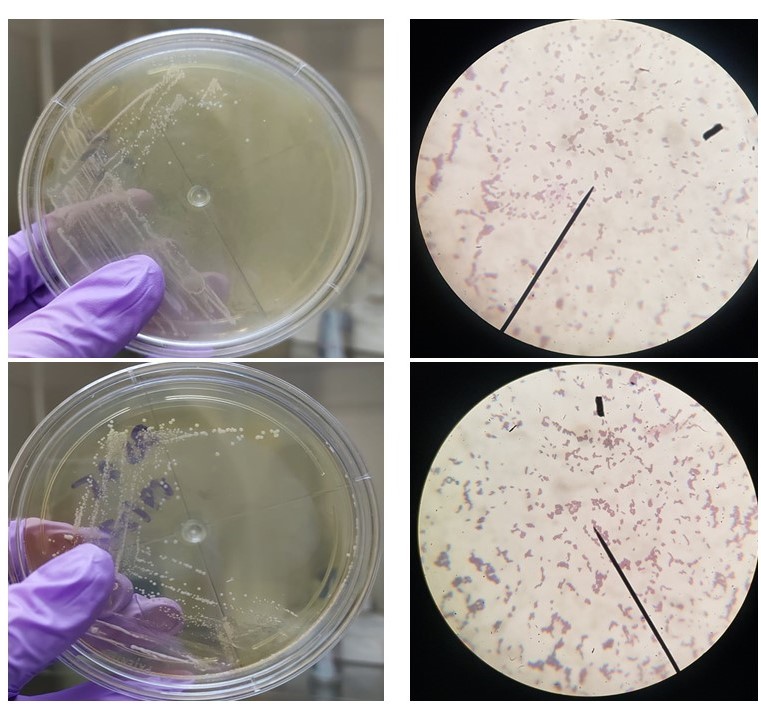
Cultural and morphological characteristics of Pediococcus acidilactici 3G3 (top) and Lactobacillus plantarum BS25 (bottom).
Related Publications
Project Instruments

FREEZONE 4.5L Benchtop Freeze Dryer
Multi-freeze drying system with a cooling trap temperature of -50 °C; ice capacity of 3 kg; teflon-coated with digital display and time control including a vacuum hose and 2 clamps; with acrylic chamber trunk and top with 8 rubber valves; with a stainless steel rack with 3 sets of stainless steel shelves and trays; with vacuum rotary vane pump and oil mist trap; inclusive of suitable fast freeze flasks, ampoules, serum bottles, ampoule pods, adapters and AVR/UPS

BIOTEK 800 TS Microplate Reader w/ Gen5 Software & Computer
UV/Vis absorbance spectrum detection mode, with spectral range of 220-1000nm; with selectable spectral resolution up to 10nm; OD range from 0 to 4 OD, accuracy <1% at 2 OD; with endpoint and kinetic measurement modes and spectral scanning; compatible with 96-well plates and standard cuvettes (10 mm path length); user definable microplate formats; can measure low-volume microspots (2uL); CCD spectrometer; with linear, orbital, and double-orbital shaking with user-definable time and speed; incubation temperature of ambient +3°C up to 45°C; with gas vent system to inject an atmosphere or to pull a vacuum into a reader; with multi-user reader control and MARS data analysis software included; USB 2.0 compatible to USB 1.1; desktop computer and compatible AVR included
DLAB Accurate 96 is based on a global vision of product design concepts and manufacturing processes. It creatively combines Fresnel lens optical signal acquisition technology, time-resolved signal separation technology and unique temperature control technology. And it reaches an international advanced level in sensitivity, multi-color crosstalk, temperature uniformity and accuracy. It supports the application of all common QPCR detection modes.
It has up to 6 fluorescence detection channels allowing multiplex PCR; effectively reduce multi-color crosstalk and edge effect, no ROX correction required; with a new optical scanning detection system; an innovative scanning method and time-resolved signal separation technology; unique edge temperature compensation technology; and a user-friendly software.
Benchmark T5000-96E TC9639 Gradient Thermal Cycler
Has an intuitive full color touch screen control; a quick, easy and foolproof Program Wizard; fully adjustable heated lid; unique multi-format block: 0.2ml tubes or strips (96), 0.5ml tubes (39), 96 well plate (1); and optimized results with Accuris™ PCR reagents
Contact Us
Mia Beatriz C. Amoranto, MSc RMicro / Princess Alyssa S. Abid, RCh
PhilDIAMOND Project 3
pdp3@upm.edu.ph
Location: GAB 402/406 AMHERG, CAS, UP Manila
Hours of operation: 9am-5pm (Mon-Fri)
Flow Cytometry Lecture Series
Lecture 1: Foundational concepts
Lecture 2: Clinical and research application
Lecture 3: Experimental Design and Protocol
Lecture 4: Data Analysis and Gating Strategies
Coming Soon:
Phil-DIAMOND Program Lecture Series (November 2021)

What is Flow cytometry?
Flow cytometry is a powerful laboratory technique that can be used to measure multiple physical characteristics of a single cell simultaneously such as size and granularity and fluorescence. This method utilizes multiple lasers as light sources that produce scattered and fluorescent light which is then read by detectors. Flow cytometry can rapidly provide qualitative and quantitative information, regarding a wide variety of cellular properties, including DNA content, surface molecule expression, cytoplasmic and nuclear antigen expression, receptor-ligand interactions, chromosome analysis, phagocytosis, target-effector cell binding, ion fluxes, expression of genes or gene products, and various light scattering properties.
Immunophenotyping using flow cytometry can be performed to identify and quantify population of cells in a heterogenous sample such as peripheral blood mononuclear cells. In our current project, UPM Flow Cytometry Core Facility will be used for immunophenotyping of HIV+ and HIV- cells isolated from Filipino participants. Human samples collected will be analyzed and sorted to characterize immune cell subpopulations of interest by labeling population-specific proteins with fluorochrome-conjugated antibodies of the surface or intracellular antigens.
The purpose of UPM Flow Cytometry Core Facility is to provide services to all research teams in need of training on the self-service cytometers (analyzer and sorter) and related software for data acquisition and analysis.
Program Instruments

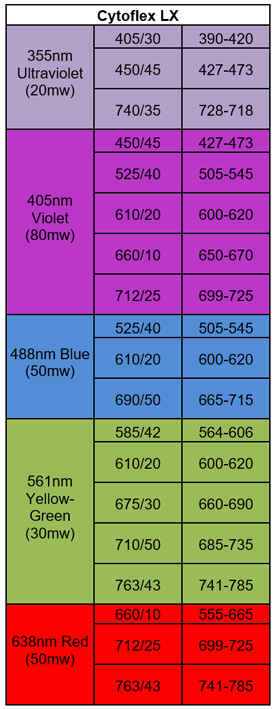
CytoFLEX flow cytometer is an analyzer that is capable of measuring 21 parameters including 19 for fluorescence detection (see Table 1). This instrument can be used to measure multiple characteristics of different types of samples in single cell suspension such as cells and different microorganisms.
Table 1. Laser and filter Configuration for Cytoflex LX
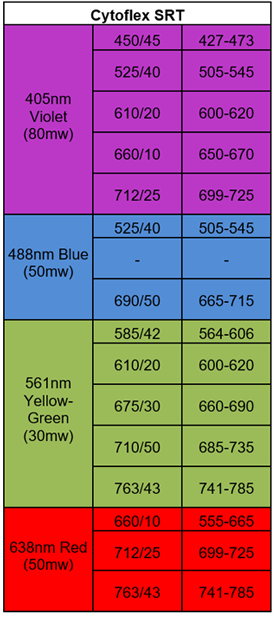
CytoFLEX SRT is a benchtop cell sorter capable of sorting a wide range of samples. It has 4 lasers that can detect 15 fluorescence simultaneously (Table 2). This instrument can be used to physically isolate cells of interest up to 4-way collection tubes as well as sort into 96-well plates. This instrument is set-up in a BSC Class II which is ideal for sorted cells that are needed to be kept sterile and alive for the experimental protocols.
Table 2. Laser and filter Configuration for Cytoflex SRT
Services
The UPM Flow Cytometry Core Facility aims at providing to its users different types of expertise:
- Cell Sorting
- Personalized training on the self-serve cytometer
- Advice on cell preparation and choice of fluorochromes
- Support for data acquisition and analysis
- Software training (Flowjo)
Contact Us
Ana Joy Padua, MD, PhD
appadua@up.edu.ph
Location: UPCPH Virology Laboratory
Hours of operation: 9am-5pm
Laboratory Services of Project Implementers/Collaborators

1. Applied Microbiology for Health and Environment Research (AMHERG)
Department of Biology, College of Arts and Sciences, UP Manila
Upcoming Services:
Freeze Drying
Equipment Use: Microplate reader (for ELISA, protein and other endpoint assays, kinetics and cell-based assays)
Contact Us:
Mia Beatriz C. Amoranto, MSc RMicro / Princess Alyssa S. Abid, RCh
pdp3.upm@up.edu.ph
(632) 8526 5861

2. Mammalian Cell Culture Laboratory (MCCL)
Institute of Biology, College of Science, UP Diliman
MTT Cytotoxicity Assay
DPPH Free Radical Scavenging Assay
Phytochemical Screening
Equipment Use: Rotary Evaporator, BSC Class II, Inverted Microscope, CO2 Incubator, Liquid Nitrogen Tank
Contact Us:
Mammalian Cell Culture Service Laboratory, Institute of Biology
mccl.upd@up.edu.ph
(02) 8981 8599 local 3729
Visit our Site:
Mammalian Cell Culture Laboratory, Institute of Biology