Click here to read more about the UPCM-PGH Harmonized Research Agenda Document
About Research Implementation and Development Office
The Research Implementation and Development Office (RIDO) primarily functions as the research arm of the College of Medicine under the supervision of the Office of the Dean and Associate Dean for Research. It aims to manage researches and its related processes to strengthen the College’s efforts towards an enhanced research environment.
Under the oversight of the RIDO Chief, the office is composed of the Technical Review Board (TRB) members from the different departments/units of the College and of the RIDO Research Working Groups for researches commissioned by the Office of the Dean. The TRB members ensure that research protocols by the College of Medicine’s constituents, as well as non-UPCM researchers, are evaluated according to their relevance and scientific merit, and are duly endorsed by the concerned department/unit prior to submission for ethics review. RIDO is likewise mandated to facilitate dissemination of research findings to the scientific community through publication and presentation in the annual research fora for faculty and for students.
RIDO previously housed both the Technical and Ethical Review Boards of the UPCM and PGH. The transition came in 2010 when the University of the Philippines Manila Research Ethics Board (UPMREB) was created to unify and integrate all existing ethical review committees. UPMREB is now the single research ethics board of the University. Nonetheless, RIDO strives to improve procedures in technical review and other research-related activities in accordance to the College of Medicine’s goals and objectives.
Organizational Structure The Office of the Dean appoints the RIDO Chief to maintain the research structure of the College, following the authority level specified in the figure below:
Organizational Structure
The Office of the Dean appoints the RIDO Chief to maintain the research structure of the College, following the authority level specified in the figure below:
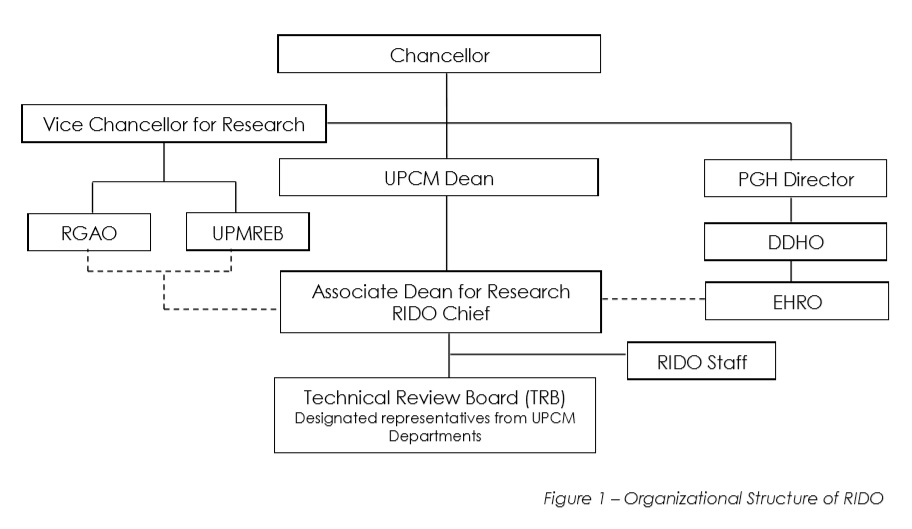 Click here to read more about the Organization Structure of RIDO.
Click here to read more about the Organization Structure of RIDO.History of Research at UPCM and PGH
Organized and structured research in UP Manila started with the creation of the Committee on Research Ethics and Development (CRED) in 1979. The CRED was initially created to manage a specific research fund with a grant from the China Medical Board. In 1984, the CRED was reorganized and became known as the Committee on Research Implementation and Development (CRID). The technical and ethics review boards were created in compliance with the set guidelines of the World Health Organization.
The National Institutes of Health, on the other hand, was created in 1996 as an implementation of a law to have a national resource center for health research. The NIH was tasked to sustain health sciences education and training, as well as to integrate existing research structures of UP Manila. It has its own Institutional Review Board which consisted of a technical and ethics review boards.
In July 2002, the CRID was registered and accredited by the Office of Human Research Protection (OHRP) of the United States Department of Health and Human Services (DHHS), with an accreditation number IRB 00002648 (CRID) U Philippines Coll Med IRB # 1. Such allowed research with US federal funding to be evaluated and eventually be conducted in the UP College of Medicine (UPCM), the Philippine General Hospital (PGH), and the Department of Medical Microbiology and the Department of Parasitology at the UP College of Public Health.
In July 2003, the CRID was upgraded from a committee to an office of the UPCM, thereby changing its name to the Research Implementation and Development Office (RIDO), which catered the Technical and Ethical Review Boards of the UPCM and PGH.
Meanwhile, in 2006, the Expanded Hospital Research Office (EHRO) was created to coordinate conduct of research at the Philippine General Hospital. EHRO then established its own Ethics Review Board to handle increasing number of research protocols of the hospital’s fellows and residents, including its nursing and research staff.
The Forum for Ethics Review Committees in Asia and the Pacific (FERCAP) gave recognition to the Ethics Review Boards of RIDO, NIH and EHRO, in addition to the registration with the Philippine Health Research Ethics Board (PHREB). In 2007, RIDO ERB was registered in the Philippine National Health Research System (PNHRS) which enlisted RIDO ERB to the Bureau of Food and Drugs (BFAD) List of Resources for Ethics Review for Clinical Drug Trials in the Philippines.
In 2010, the ethics review function of RIDO was transferred to the newly created UP Manila Research Ethics Review Board (UPMREB) by virtue of an administrative order of Chancellor Ramon Arcadio, integrating all existing ethics committees in UP Manila under a single research ethics board.
Technical Review Guidelines, and Forms
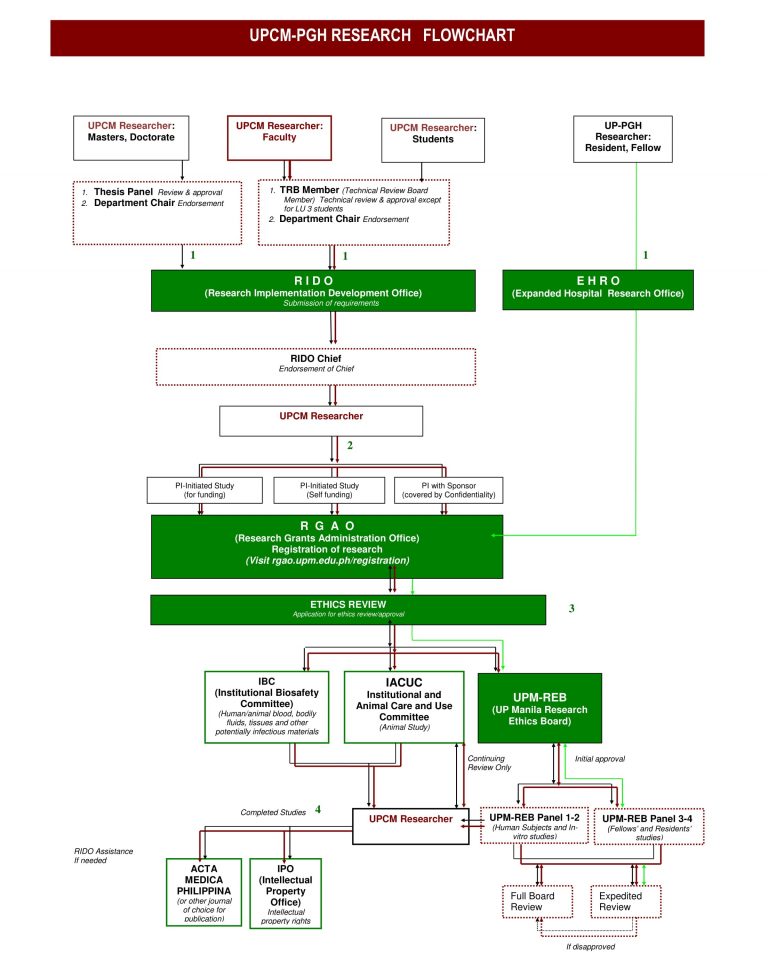
Research Flowcharts
UPCM Process for Technical & Ethical ApprovalProcedures
Technical Review of Research Protocols CM Constituents as Research Subjects/ParticipantsTechnical Review Board (TRB) Members/Reviewers
Technical Review BoardRIDO Forms
Technical Review Form Principal Investigator’s Response to Technical Review Form Research Registration Form UPCM RIDO Form 5 Final Report Form UPCM Researcher Guide Progress ReportContact Us
PM203, Paz Mendoza Memorial Bldg, College of Medicine
547 Pedro Gil Street, Ermita, Manila
Mobile Phone No: +639297525048
Email: upm-rido@up.edu.ph
UPM Research Ethics Board: Kindly click here
